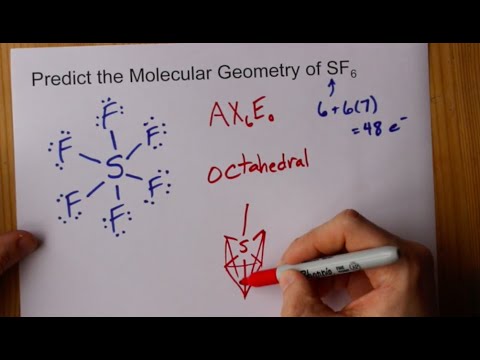
Geometry of SF6 is ?
Geometry of SF6 is ?
Explanation
The molecular Geometry of SF6 (sulfur hexafluoride) is octahedral.
In this molecule, the central atom i.e. sulphur has a total of six electrons in its valence shell
And in the molecule, we have six fluorine atoms so it shares one electron with each fluorine atom.
So this molecule has six bond pairs and no lone pair.
So its structure is octahedral
The bond angles in sulfur hexafluoride molecules are 90A∘ and 180A∘.